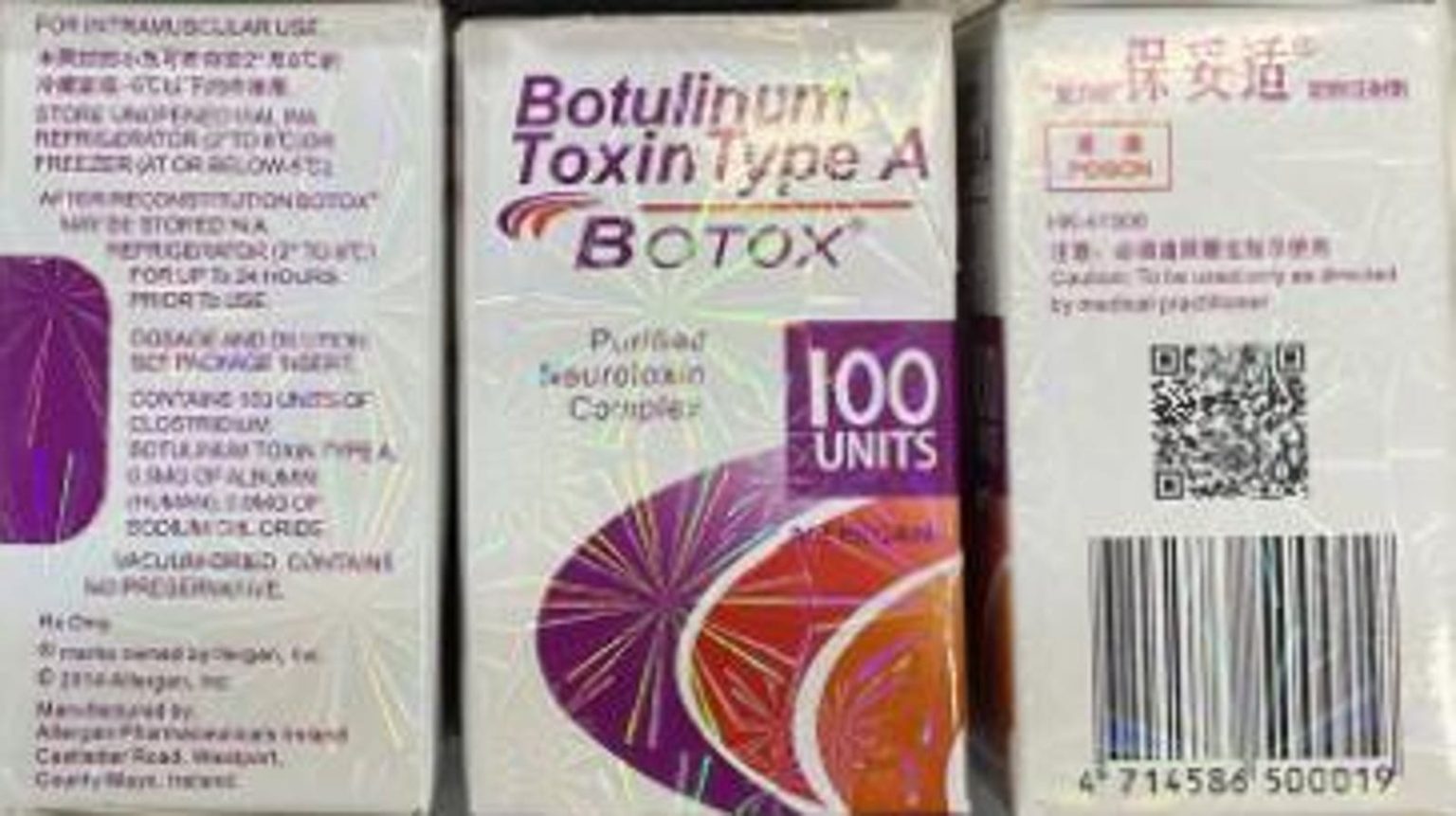
The CDC and FDA have launched an investigation into counterfeit Botox injections that have caused nearly 20 adverse reactions in nine states, leading to warnings about the dangers of using unlicensed providers and products. These cases were reported in states like Colorado, Florida, and New York, with some patients requiring hospitalization and treatment for symptoms resembling botulism. All affected individuals were women in their late 30s seeking cosmetic enhancements with Botox, with symptoms including blurred vision, difficulty swallowing, and weakness, among others.
Symptoms of the fake Botox injections could also include fatigue, droopy eyelids, and slurred speech, with potential complications such as botulism, a rare but serious illness affecting the nervous system. While the chances of dying from botulism are relatively low if treated properly, the FDA and CDC have yet to determine the source of the counterfeit products. Authentic Botox is only manufactured by AbbVie or Allergan, and the agencies have highlighted signs of a fake product, such as errors in labeling and packaging, to help the public identify potential risks.
Botox, originally approved by the FDA in 1989 for certain eye disorders, has since gained popularity for cosmetic use in treating wrinkles. The average cost per injection can vary depending on the amount and area treated, with effects lasting for a few months before the muscles regain movement and wrinkles reappear. Made from a safe bacterium in small amounts but toxic in large doses, Botox works by blocking nerve signals that cause muscle contractions, leading to smoother skin in the treated area. Despite the recent incidents with counterfeit Botox, the FDA assures the public that genuine products from AbbVie and Allergan are safe and effective when used correctly.
In a previous crackdown on unregulated cosmetic treatments, U.S. officials seized nearly 80 shipments of unapproved injectable cosmetics valued at over $175,000 in Cincinnati. These products were being imported from various countries and distributed to states including New York, Florida, and Oregon. Given the risks associated with counterfeit treatments, health authorities continue to investigate the current cases of adverse reactions linked to fake Botox to ensure public safety and prevent further harm. The FDA and CDC are working with state health departments to identify the source of the counterfeit products and take necessary actions to protect consumers from potentially harmful consequences.
Overall, while Botox injections have become a popular cosmetic treatment for reducing wrinkles and fine lines, the recent reports of adverse reactions to counterfeit products highlight the importance of using licensed providers and authentic medications. Patients seeking Botox treatments should be vigilant about verifying the source and authenticity of the products used to minimize the risks of complications and ensure safe and effective results. As investigations into the counterfeit Botox cases continue, the FDA and CDC are committed to raising awareness about the potential dangers of unregulated treatments and providing guidance to healthcare professionals and consumers on best practices for cosmetic procedures.